Evaluating the Pharmaceutical Sector Through Pharmacopoeial Standards: A Review
Keywords:
pharmaceuticalAbstract
For the pharmaceutical tablet to be considered a standard drug approval, it must fulfill
certain requirements. Various standard factors, including identification, strength, quality,
purity, and stability, are used by pharmaceutical companies to test tablets for accuracy. For
this reason, pharmaceutical procedures must be controlled, regardless of the problems they
may resolve. Raw material inspection, process control, and final product targeting are all
included in process control. For this reason, it is important to keep an eye on how well
process control is working. In this regard, the manufacturing process should be modified
in accordance with the specifications as required, which may also include environmental
and equipment management. During the production process, the quality control unit should
accept or reject pharmaceutical items after properly examining them for identification,
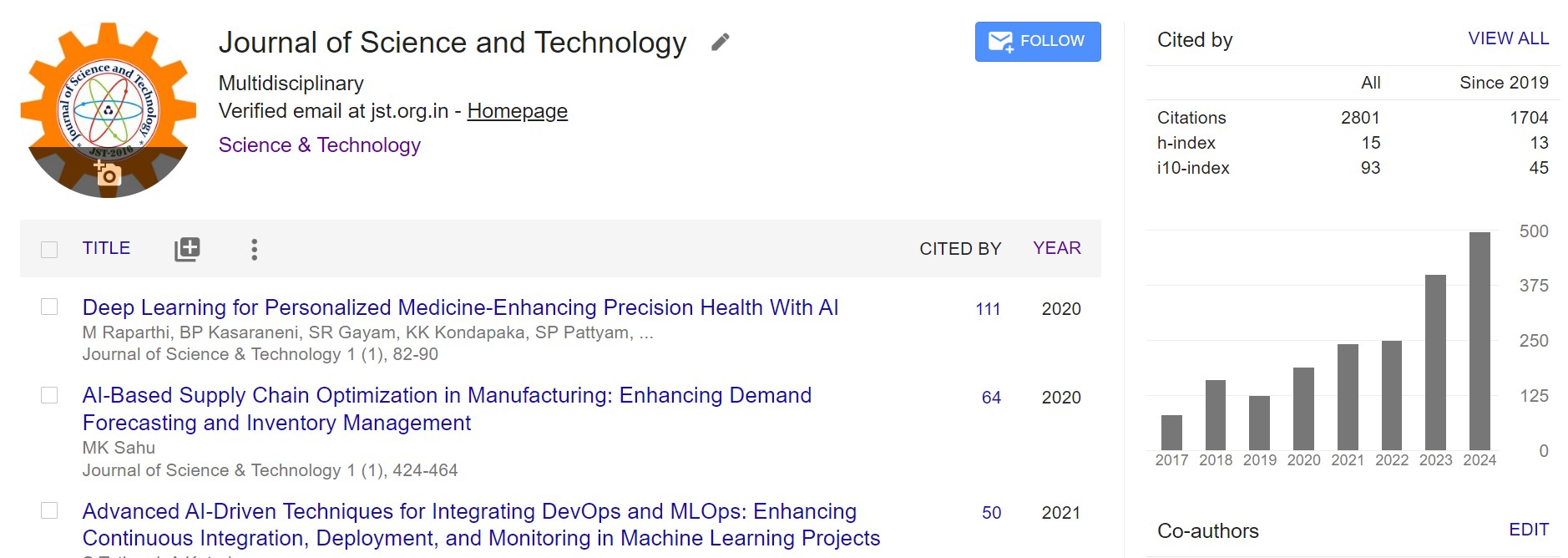
strength, quality, and purity. Highlights of this study include describing pharmaceutical
product quality control testing utilizing various equipment for the pharmaceutical sector in
accordance with pharmacopeias.