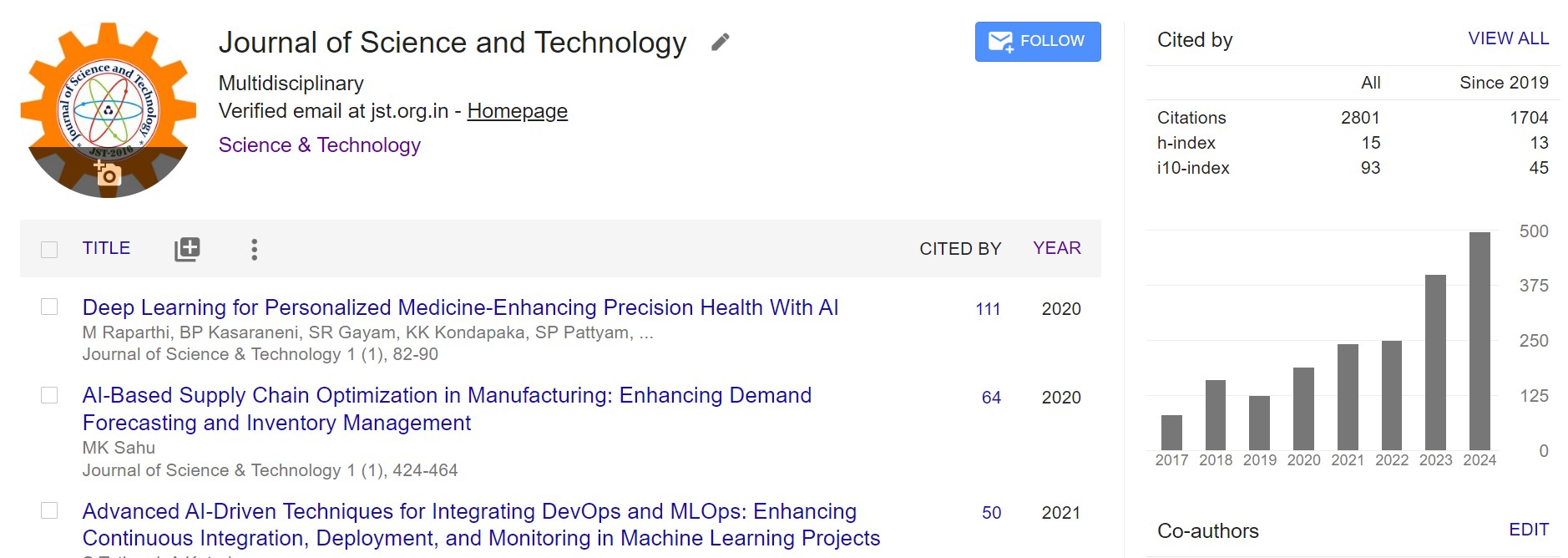
Designing a Nitrosamines-Free Controlled Release Matrix Tablet of Paliperidone: A Quality by Design Approach
Keywords:
ROS-based extended-releaseAbstract
Paliperidone, a psychiatric medication belonging to the atypical antipsychotic family, is the 9-hydroxy metabolite of risperidone.
The racemates of paliperidone are (+)- and (-)-paliperidone. It functions centrally as a dopamine D2 antagonist with
serotonergic 5-HT2A antagonistic activity. Invega ER tablets are made using ALZA OROS® osmotic drug release technology.
Paliperidone is administered in a specific manner over a 24-hour period using this tri-layer longitudinally compressed tablet,
which is based on an advanced osmotic administration mechanism. The goal of this study is to develop a generic paliperidone
controlled-release single-layer matrix tablet. In order to help create a stable and robust formulation, several combinations of
Polyox and hypromellose were utilized in the core, followed by coating. The in-vitro disintegration characteristics of all strengths
are comparable. Both the challenge for the alcohol dosage dumping research and the nitrosamine risk assessment were
evaluated for the freeze formulation. With a pKa1 of 8.2 for the piperidine moiety and a pKa2 of 2.6 for the pyrimidine moiety,
paliperidone is a basic chemical. Consequently, at healthy pH, a significant amount of the molecule is ionized. At a pH of 7.4, it is
comparatively insoluble in water (0.003 g/100 mL). The solubility dramatically rises at lower pH (3 g/100 mL at pH 5.3) and
falls at higher pH (0.001 g/100 mL at pH 12.9). Octanol/water's partition coefficient (log P) is 2.39. As a result, pH 2.75 buffer
was determined to be the discriminating medium. The in-vitro release profile was expressed using matrix composition and the
Higuchi model. In vitro release experiments show that the formulation can resist alcoholic conditions ranging from 0% to 40%.
It is stable, affordable, and simple to formulate. In order to minimize the chemical interaction between an active ingredient and
other excipients, the production process consists of dry mixing, compression, and coating. Therefore, the formulation has a very
little chance of producing nitrosamine impurities. When it comes to dosage dumping, the formulation is categorized as tough.