Design and In-vivo Assessment of Quercetin-Based Nanosponges Buccal Quercetin Tablets
DOI:
https://doi.org/10.46243/jst.2021.v6.i04.pp292-301Keywords:
.Abstract
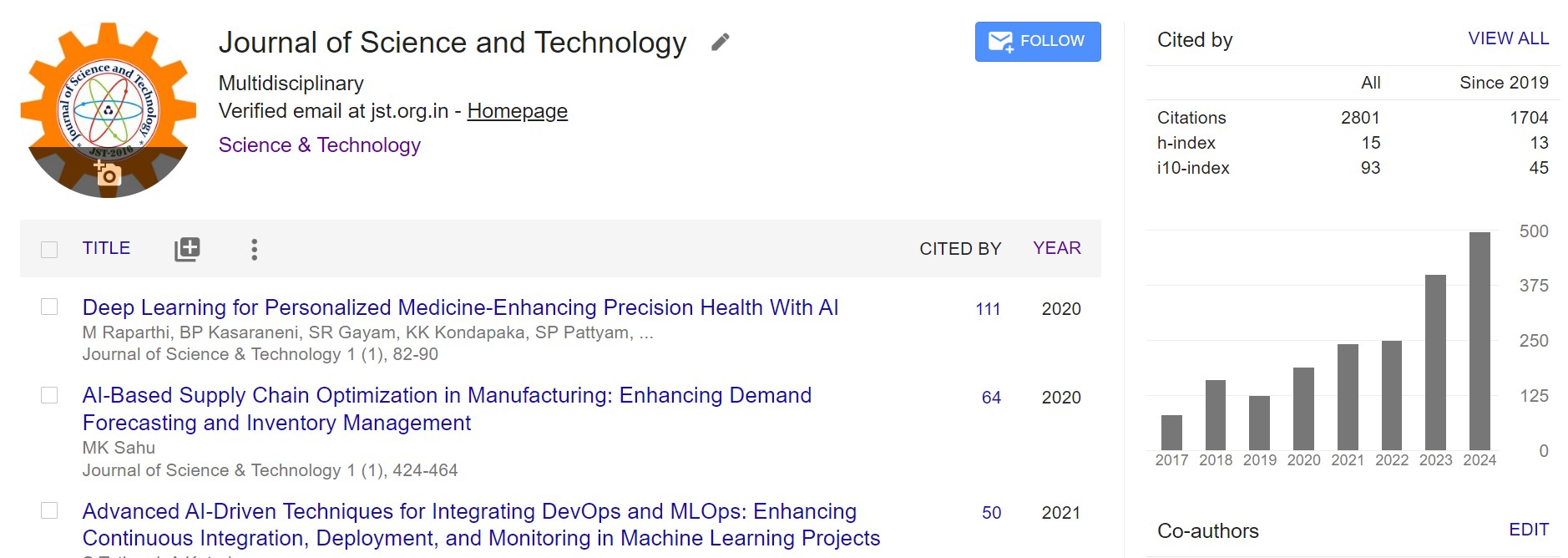
The goal was to use cyclodextrin-based nanosponges to create a controlled release formulation that would boost quercetin's bioavailability. Using the freeze-drying method, a 3-factor, 3-level Box-Behnken design containing quercetin was loaded into nanosponges based on the results of preliminary testing. After being manufactured, characterized, and formed into tablets, the prepared nanosponges were inspected. The drug release percentages at six hours range from 53.04 to 82.64% for the quercetin-loaded nanosponges, whereas the particle sizes range from 36.45 to 135.27 nm and the encapsulation efficiencies from 42.37 to 88.44%. The Quercetin-nanosponge interaction was confirmed by FTIR, DSC, and XRD analyses. After the nanosponges were converted into tablets, the medication released from them at a rate of 99.75% in vitro, and stability tests revealed no appreciable alterations six months later. Rats were used in in vivo investigations to compare the Cmax of quercetin optimized nanosponges tablets (6.27 ± 0.06 ng/mL) to the Cmax of the pure medication (3.07 ± 0.086 ng/mL), which was substantially lower (p<0.05). The Tmax values for the pure drug solution and the nanosponges tablet formulation were 0.5 ± 0.08 h and 4.0 ± 0.07 h, respectively.